Please choose the topic you would like to learn more about:
Aortic Dissection
Description
The Aorta is the largest artery in the body and carries oxygen-rich blood from the left heart to the entire body. It is curved like a candy cane and is divided in three segments: the ascending aorta (the initial, upwardly directed part) from which the blood vessels to the heart arise, the arch which gives out the blood vessels to the head and upper arms, and the descending aorta to all other blood vessels in the rest of the body. The wall of the aorta has three layers of tissue, and is made to withstand the pressure build-up as blood travels from the left heart to the various blood vessels.
Aortic dissection begins from a defect (“tear”) in the innermost layer (called the “intima”) of the aortic wall. Another cause of aortic dissection is bleeding from a small feeding vessel to the aortic wall. From the tear, blood enters between the layers of the aortic wall and separates the layers. That results in the formation of a second channel, through which blood can pass. The separation of the aortic wall layers causes blood to travel inside both the original (“true”) and the newly formed (“false”) channels (or lumens). Blood traveling inside the false lumen is diverted away from the body vessels, and the decreased blood may result in failure of body functions.
Symptoms
A patient suffering from aortic dissection will usually feel a sharp pain anywhere in the chest, in the back between the shoulder blades or even at the belly. As the aortic dissection spreads throughout the length of the aorta, symptoms related to heart attack, stroke, kidney failure, bowel malfunction or leg paralysis can develop.
Causes
Atherosclerosis (hardening of the arteries), high blood pressure, connective tissue disease such as Marfan’s or Ehlers-Danlos syndrome or congenital problems of the aortic valve, such as a bicuspid aortic valve, are some of the risk factors that may predispose one to suffer an aortic dissection.
Diagnosis
The diagnosis is done with chest x-ray , with CT-scan or MRI and with echocardiography.
Transesophageal echocardiography is considered the safest and fastest bed-side technique to check for the presence of a second lumen inside the aorta quickly. The echocardiographer can see the two channels that the blood is travelling, the location of the initial damage (“tear”) of the wall as well as how far the dissection has spread down the aortic tube.
Treatment
If aortic dissection starts at the ascending aorta or involves the arch, the only option is surgical repair of the aortic wall, usually by replacing the damaged wall with synthetic material. If the dissection is limited in the descending aorta, blood pressure regulation with medications in the intensive care unit can limit its spread. Over the long term, the weakened aortic wall may balloon and form an aneurysm, which may be prone to rupture. Therefore, lifelong follow up and evaluation is necessary.
Aortic stenosis
Aortic valve stenosis is a condition in which the aortic valve is deformed and narrowed. This process can worsen over time.
Cause
Common causes of this condition include:
- Congenital abnormalities – an abnormality that has been present since birth
- The most common congenital abnormality is a bicuspid aortic valve. This is where there are only two leaflets of the valve instead of three.
- Rheumatic Fever
- Age-related wear and tear of the valve
Symptoms
A patient with severe aortic stenosis may experience:
- shortness of breath
- chest discomfort
- fainting spells
Diagnosis
Aortic stenosis can be detected by a physical examination performed by a healthcare provider. Echocardiography can diagnose aortic stenosis by showing the narrowed aortic valve. Echocardiography can be used to monitor the progression of the disease as well as the response to therapy.
Treatment
When aortic stenosis becomes severe and symptoms are present, surgery for valve replacement is the usual treatment. Other non-surgical options may exist for the pediatric population.
Aortic Regurgitation
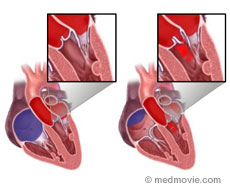 Aortic regurgitation is a leaking of the aortic valve.
Aortic regurgitation is a leaking of the aortic valve.
Cause
The most common causes of a leaky aortic valve are:
- abnormal structure
- enlargement of the aorta
- Rarely infections of the lining of the aortic valve.
When the aortic valve cannot form a tight seal, blood will enter the left ventricle flowing backwards, from the major vessel (aorta) of the body. The looser the seal, the more the backward blood flow will be. This will cause an increase in size of the left ventricle, and can gradually lead to heart muscle failure.
Diagnosis
Echocardiography can detect a leaky aortic valve and help to determine when the valve needs to be fixed (repaired) or replaced. The abnormal blood flow through a leaky aortic valve can be seen with color echo and the backward flow inside the aorta can be evaluated with Doppler echo.
Aortic aneurysm
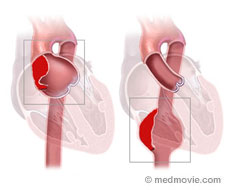 Aortic aneurysm is a term used for an enlargement in an area of the aorta that is weak. Aorta is the major blood vessel in the body that arises from the heart and runs through the center of the chest and abdomen and feeds blood through its branches to the entire body. The part of the aorta that is in the chest is called the thoracic aorta and the part that is in the abdomen is called the abdominal aorta. Normal aorta is 2.5-3cm in diameter. The aneurysm initially starts as a small bulge in an area of the aorta that is weak but over a period of time gradually increases in size. If this abnormality is in the part of the aorta that is in the chest it is called thoracic aortic aneurysm and if this develops in the segment of the aorta that is in the abdomen it is called abdominal aortic aneurysm (AAA)
Aortic aneurysm is a term used for an enlargement in an area of the aorta that is weak. Aorta is the major blood vessel in the body that arises from the heart and runs through the center of the chest and abdomen and feeds blood through its branches to the entire body. The part of the aorta that is in the chest is called the thoracic aorta and the part that is in the abdomen is called the abdominal aorta. Normal aorta is 2.5-3cm in diameter. The aneurysm initially starts as a small bulge in an area of the aorta that is weak but over a period of time gradually increases in size. If this abnormality is in the part of the aorta that is in the chest it is called thoracic aortic aneurysm and if this develops in the segment of the aorta that is in the abdomen it is called abdominal aortic aneurysm (AAA)
Cause
Thoracic aortic aneurysms most often result from a process called cystic medial degeneration which is often an age-related change (usually in the 6th and 7th decade of life) in the wall of the aorta that leads to weakening and formation of the aneurysm. Presence of high blood pressure increases this risk. When it occurs in young patients, it is most often due to Marfan’s syndrome (which is an inborn defect in the connective tissue protein that the wall of the aorta is made of) or other less common connective tissue disorders such as Ehlers Danlos syndrome. Patients with Bicuspid aortic valve can also have associated aortic aneurysms of the ascending aorta. Atherosclerosis or the hardening of the arteries is associated with majority of the aneurysms in older patients. The risk factors for aneurysm formation are the same as those for atherosclerosis. However, it remains unclear if atherosclerosis is actually responsible for aneurysm formation. Certain types of infections as well the diseases that cause inflammation of the wall of the aorta can also cause aneurysm by causing weakness in the aortic wall. Other individuals at risk for aortic aneurysms include those with connective tissue disorders such as Marfan’s Syndrome or Ehlers Danlos syndrome. Certain congenital defects such as bicuspid aortic valves also increase the risk of aneurysm formation.
Symptoms
Patients with aortic aneurysm usually have no symptoms and in fact may not even be aware of the problem. People can live with this problem for years without any symptoms. However, when the aneurysm gets big patients can develop chest, abdominal and back pain. Sudden onset of severe chest, abdominal and back pain usually indicates aneurysm rupture which is a surgical emergency. Thoracic aneurysm is often diagnosed in an asymptomatic patient on a chest x-ray or echocardiogram that is done for other reasons. Rarely patients may experience symptoms of hoarseness, difficulty in swallowing, wheezing, cough, etc. resulting from the aneurysm compressing the neighboring structures. Abdominal aortic aneurysm is often incidentally noted by the health care provider that examines the patient during a routine physical in the form of an abnormal pulsation in the abdomen. Alternatively, this may be noted for the first time on an abdominal ultrasound that is performed for some other reason.
Diagnosis
Usually an abnormal CXR suggests the diagnosis of a possible thoracic aneurysm. An echocardiogram however is very helpful to confirm the diagnosis.
Echocardiograms can show:
• The location and extent of the aneurysm,
• The size of the aneurysm
• If there is associated aortic insufficiency
• The effect of the leak on the heart
A CT scan and magnetic resonance imaging are other imaging technologies available to diagnose aortic aneurysms.
Treatment
Once the diagnosis is made with the help of the echocardiogram, the size of the aneurysm over time is monitored by serial echocardiograms, CT scans, or MRI. Alternatively, both the initial diagnosis as well as the serial follow up can be done with either CT or magnetic resonance imaging. Decision is made to intervene with treatment once the aneurysm reaches a certain size (beyond which risk of rupture increases). The aneurysm is rapidly increasing in size, or when the patient becomes symptomatic. The treatment is usually an operation called aneurysmectomy with graft repair. Sometimes they can be treated with special stents. A transesophageal echocardiogram in the operating room can help in guiding the surgery and can also evaluate the effectiveness of the repair.
Atrial Septal Defect
 Background
Background
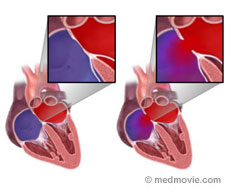
The normal human heart contains four chambers. The upper chambers are known as the left atrium and the right atrium. The left atrium collects blood as it returns to the heart from the lungs, after picking up oxygen (“red blood”). The right atrium collects the low oxygen (“blue”) blood as it returns to the heart from the body. The wall that separates the left atrium from the right atrium is known as the atrial septum.
Normally, there is no opening in the atrial septum, so that the low oxygen blood remains on the right side of the heart, and the high oxygen blood remains on the left side of the heart. However, in approximately 1 in 1000 births, there is a hole in that wall known as an atrial septal defect (ASD). This should not be confused with a patent foramen ovale (PFO), which is a normal opening in the atrial septum that is important for the circulation of blood in a fetus, and which usually closes early in life. An atrial septal defect is usually seen in an otherwise normal heart, but can also be commonly seen in people who have other congenital heart defects.
Symptoms
When an ASD is present, blood typically flows from the left atrium into the right atrium. This causes extra blood to flow through the right side of the heart and be pumped into the lungs. Over time, this can cause enlargement of the right side of the heart, due to the extra blood flow. Children with an ASD typically have no symptoms, although they can develop shortness of breath with activity later in childhood. Other symptoms in children are quite rare. However, people who have an ASD often develop problems in adulthood, including congestive heart failure, abnormal, fast heart rhythms (atrial fibrillation or atrial flutter), or rarely, abnormally high blood pressure in the lungs.
Diagnosis
Due to the usual lack of symptoms, an ASD is usually diagnosed after a routine physical exam; the health care provider may hear a heart murmur or a change in the heart sounds that is suggestive of the diagnosis. If an ASD is suspected, a referral to a pediatric cardiologist may be recommended, and an echocardiogram (heart ultrasound) may be performed. The echocardiogram provides pictures of the atrial septum, directly showing its structure and whether there is an ASD present.
Questions the echocardiogram will address include:
- Size and location of the ASD
- Presence of multiple ASDs
- Blood flow across the ASD
- Presence of enlargement of the right side of the heart
Occasionally, particularly in adults, the transthoracic echocardiogram will not show pictures clear enough to make the diagnosis. In that situation, a transesophageal echocardiogram may be ordered, in which a special echo probe is placed down the throat to take pictures from behind the heart.
Treatment
If an ASD is large enough or has enough flow across it to cause the right side of the heart to enlarge, your physician will likely recommend that it be closed. This may be recommended even if there are no symptoms, in order to prevent problems from occurring later in life. There are two different methods that may be recommended for the closure of an ASD:
- Surgery: a heart surgeon will close the ASD directly, either by sewing it closed primarily, or by sewing a patch into the wall to cover the hole.
- Device closure: a cardiologist will access the heart through a vein, typically located at the top of the leg. A catheter is placed across the ASD, and a device is placed in the heart that straddles the hole with arms or disks on both sides, effectively closing the defect.
A transesophageal echocardiogram is often used in the operating room to be sure that the ASD is closed after surgery, or is used to help guide the placement of the ASD closure device in the catheterization laboratory.
Cardiomyopathies
The heart is made up of three general parts. An electrical system that regulates the heart’s beating, a “plumbing” system comprised of the coronary arteries which transport blood to nourish the heart muscle so that it has energy to beat, and a muscular component which does the work of pumping blood around the body. The heart is made up of 4 chambers: a left atrium and ventricle and a right atrium and ventricle. The left sided chamber (ventricle) pumps oxygenated blood to the body via arteries and the right-sided chamber gathers deoxygenated blood from the veins and pumps it to the lungs. Normal cardiac system image.
Cardiomyopathy means disease of the heart muscle, called the myocardium. There are different types of cardiomyopathies which are categorized by the structural and functional abnormalities that occur within the heart muscle.
Dilated Cardiomyopathy
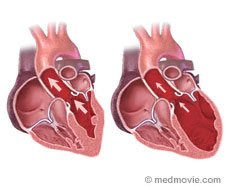 In dilated cardiomyopathy (DCM) the heart becomes enlarged and weakened.
In dilated cardiomyopathy (DCM) the heart becomes enlarged and weakened.
Causes
DCM has many causes but the resulting detrimental effects on the structure and function of the heart are the same. Common causes include:
-
- inherited cardiac muscle defects
- toxins such as alcohol
- abnormal reactions to a common virus
- thyroid disorders
Symptoms
In DCM, the left ventricular and sometimes right ventricular muscle is weakened and cannot supply adequate oxygen and nutrients to the body. When this is mild, a patient would feel the effects only when they need an increase in energy such as walking up stairs. This could make them feel easily tired and short of breath. When the muscle is severely weakened, the patient may feel poorly even at rest. Sometimes in DCM, the patient’s body holds on to extra fluid that backs up into the lungs or legs and causes swelling; this is called congestive heart failure.
Diagnosis
Echocardiograms can diagnose DCM. The ventricular chamber appears enlarged and weakened; the muscle does not thicken and contract very strongly. As the chambers dilate, the supporting structure of the valves, which separate the atrial from ventricular chambers, become stretched and do not close properly. This leads to mitral and tricuspid regurgitation Echocardiograms are an excellent way to monitor patients with DCM. Once treatment is started, Doctors can assess the effects of medications on the heart’s size and function.
DCM is characterized by cardiac muscle cell damage and loss. This can begin slowly and progress over years or it may be very aggressively and present within weeks and very acute.
Treatment
DCM is a chronic condition with a waxing and waning course. With medications, the strength of the heart may improve, resulting in the patient feeling better.
Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy is when the heart muscle is abnormally thickened due to enlargement of the muscle fibers. This thickening is called hypertrophy. One example of hypertrophy is the muscular enlargement seen in bodybuilders from lifting weights. Hypertrophic cardiomyopathy (HCM) however, is an abnormal thickening of the muscular walls of left ventricle, the chamber which pumps blood around the body.
Cause
It can occur in older patients from the increased workload the heart performs pumping against high blood pressure for many years. However, HCM most commonly results from one of several genetic abnormalities that cause the heart muscle to grow too thick. In these cases, the disease can manifest in young adults or even children. 1 in 500 people have HCM and it may be inherited within families. Unlike dilated cardiomyopathy where the squeezing of the heart is weakened, in HCM the squeezing of the left ventricle is usually too strong.
HCM has different forms depending on how much and what portion of the left ventricle is affected. Hypertrophy can involve the entire left ventricle or be localized to a specific area such as the mid-ventricle or the bottom portion of the left ventricle called the apex.
The most common form affects the septum or wall between the right and left ventricles below the aortic valve. This form sometimes results in obstruction of blood being ejected out of the left ventricle into the aorta. Here a leaflet of the mitral valves is sucked up against the septum as the heart squeezes. This is termed hypertrophic obstructive cardiomyopathy (HOCM).
Symptoms
Patients with HCM may have no symptoms at all or may experience:
- shortness of breath on exertion
- chest pain
- Palpitations.
Congestive heart failure can develop because the abnormal thickening of the heart muscle prevents the heart from relaxing normally. This leads to increased pressure in the cardiac chambers and congestion in the lungs and body. Some patients with HCM are at risk of developing abnormal heart rhythms, called arrhythmias that may cause sudden death.
Diagnosis
Patients with HCM frequently have abnormalities on the electrocardiogram (ECG), which measures the electrical activity of the heart; however, the primary test to diagnose HCM is echocardiography. Echo demonstrates the typical features of HCM and determines which variant is present. Echo Doppler can follow changes with treatment over time. Echo also allows for the evaluation of additional cardiac abnormalities such as disease of the valves. Genetic tests are being developed which may be used in the future to diagnose HCM, provide information about prognosis and screen asymptomatic family members.
Treatment
Treatment of HCM depends on the symptoms present. Medications such as beta blockers and calcium channel blockers are used to reduce obstruction in patients with HOCM, improve symptoms and control arrhythmias. In cases of severe obstructive cardiomyopathy not controlled with medications, surgical procedures can be used.
Myectomy is an open-heart surgery where a portion of the hypertrophied septum is cut away to relieve obstruction. A less invasive procedure, alcohol septal ablation, can be utilized in patients who are not candidates for surgery. This is done under sedation in the cardiac catheterization laboratory. Here catheters deliver a small amount of ethanol alcohol into the affected area of the septum. This creates a small heart attack which can reduce the obstruction. Pacemakers and implantable defibrillators are used in patients who have dangerous heart rhythms.
Finally, first degree relatives should be screened with an echocardiogram to look evidence of the disease.
Restrictive Cardiomyopathy
Restrictive cardiomyopathies (RCM) are a group of diseases of the heart muscle that impair the heart’s ability to relax and fill with blood while the squeezing function usually remains preserved. These processes cause the heart muscle to become stiff. Stiff ventricles result in increased pressure in the heart chambers leading to congestion and the typical signs of heart failure.
RCM is a rare process. There are two major categories. Primary restrictive cardiomyopathy causes progressive fibrosis (development of extra tissue) of the heart muscle. The cause is unknown. RCM also results from the accumulation of foreign material within the myocardium (middle layer of heart muscle) as a consequence of a variety of infiltrative diseases. Examples include amyloidosis, in which abnormal proteins are deposited in the myocardium, hemochromatosis (iron overload disease), Fabry’s disease (a disease caused by the lack of or faulty enzyme needed to metabolize lipids, fat-like substances that include oils, waxes, and fatty acids), and sarcoidosis (an inflammatory disease). RCM may rarely occur in patients who’ve received radiation therapy to the chest. Restrictive cardiomyopathy is diagnosed clinically but echocardiography can be helpful in demonstrating abnormal relaxation properties of the ventricle, in excluding other forms of cardiomyopathy, and in some cases identifying features of specific diseases such as cardiac amyloidosis.
Takotsubo Cardiomyopathy (broken heart syndrome)
Takotsubo cardiomyopathy is a recently recognized condition. It has also been called the Apical Ballooning Syndrome and the Broken Heart Syndrome. The name takotsubo is Japanese for “octopus trap” and was coined in Japan where it was observed that the characteristic cardiac abnormality seen in this syndrome causes the heart to resemble an octopus trap.
Symptoms
Patients with takotsubo cardiomyopathy develop sudden cardiac dysfunction that mimics a heart attack. They may experience:
- chest pain
- abnormalities on the electrocardiogram
- Elevated levels of chemicals called troponins in the blood that represent injury to the heart muscle.
However, unlike patients with a typical heart attack who have obstructed coronary arteries, patients with takotsubo cardiomyopathy have no such blockages. Imaging tests of the heart such as echocardiography show characteristic abnormalities of squeezing of the left ventricle where the bottom portion of the heart called the apex balloons out during contraction. Patients with takotsubo cardiomyopathy may become quite ill. However, the abnormalities typically resolve after two –four weeks and follow up echocardiograms show return of normal cardiac function. The cause of takotsubo cardiomyopathy is not fully understood. It occurs more commonly in women and usually follows a severe emotional trauma which is why it is sometimes referred to as “broken heart syndrome”.
Right Ventricular Cardiomyopathy
Right ventricular cardiomyopathy, also called right ventricular dysplasia, is a rare condition.
Causes
Right Ventricular Cardiomyopathy is caused by fibrous and fatty tissue replacing the normal heart muscle predominately in the right ventricle. This is caused by acquired or inherited specific mutations of protein complexes and anchoring filaments within heart muscle cells.
Symptoms
Patients with RV dysplasia often have dangerous ventricular arrhythmias and this may be their only symptom.
Diagnosis
The diagnosis can be made with echocardiography. The right ventricle may appear dilated and/or have regions which are aneurysmal, (A localized, pathological, blood-filled dilatation of a blood vessel caused by a disease or weakening of the vessel’s wall) or do not contract normally).
Mitral Regurgitation: (Leaking of the valve)
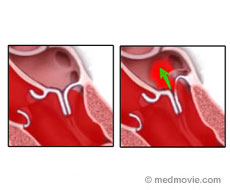 Mitral Regurgitation is a leaking of the mitral valve. The red blood (rich in oxygen) that comes back from the lungs into the left heart first enters the left atrium and moves through the mitral valve to enter the left ventricle. The left ventricle pumps blood into the aorta which then carries the blood to the entire body. During the time when the left ventricle is pumping blood into the aorta, the mitral valve remains closed to prevent the blood from coming back into the left atrium. However, if the mitral valve is not able to close properly some of this blood can flow backwards into the left atrium. This leaking of the mitral valve called, mitral regurgitation, is quite common.
Mitral Regurgitation is a leaking of the mitral valve. The red blood (rich in oxygen) that comes back from the lungs into the left heart first enters the left atrium and moves through the mitral valve to enter the left ventricle. The left ventricle pumps blood into the aorta which then carries the blood to the entire body. During the time when the left ventricle is pumping blood into the aorta, the mitral valve remains closed to prevent the blood from coming back into the left atrium. However, if the mitral valve is not able to close properly some of this blood can flow backwards into the left atrium. This leaking of the mitral valve called, mitral regurgitation, is quite common.
Cause
The most common primary valve problem that causes mitral regurgitation in the developed countries is:
- Mitral valve prolapse which is a primary defect in the connective tissue the valve is made of. In advanced cases of prolapse there could be a tear in the valve.
- Infection of the valve (endocarditis)
- Diseases of the heart muscle): When the left ventricular muscle becomes weak either from heart attack or other forms of diseases of the ventricular muscle (cardiomyopathy) the heart becomes less efficient and becomes enlarged. When this happens the valve because of its attachment to the underlying ventricle is pulled apart and has difficulty closing and therefore becomes leaky. (link to cardiomyopathy)
- Rheumatic fever, which along with causing mitral stenosis as noted above can also cause mitral regurgitation. This however is more common in the developing countries
Symptoms
People who have this problem initially have no symptoms and in fact may not even be aware of the problem. This is usually first noted by the health care provider that examines the patient with a stethoscope and hears a heart murmur. Alternatively, this may be noted for the first time on an echocardiogram that is performed for some other reason. People can live with this problem for years without any symptoms. However, the problem can get worse over time and result in enlargement of the heart. When this occurs, symptoms include:
- shortness of breath
- difficulty with exercise
- fatigue
- leg swelling
Diagnosis
An echocardiogram is the best method to diagnose this condition. Echocardiograms can show:
- If there is a leak
- The severity of the leak
- Cause of the leak
- The effect of the leak on the heart
Treatment
Once the diagnosis is made with the help of the echocardiogram, the progression of this condition over time is monitored by yearly physical exam as well as serial echocardiograms. Decision is made to intervene with treatment once the patient becomes symptomatic or develops certain changes on the echocardiogram. The treatment is usually open-heart surgery when the valve can be either repaired or replaced with an artificial valve. A transesophageal echocardiogram in the operating room can help in making this decision and can also evaluate the effectiveness of the valve surgery.
Mitral Stenosis (narrowing of the mitral valve)
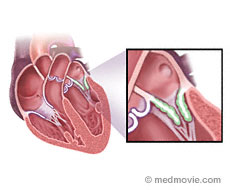 Mitral Stenosis is a narrowing of the mitral valve.
Mitral Stenosis is a narrowing of the mitral valve.
Cause
The most common cause of mitral stenosis is rheumatic fever. Most of you will remember going to the doctor as a child when you had a sore throat. The doctor often would check for “strep” (streptococcus) and then decide whether to treat with antibiotics. There is a small risk that if strep is left untreated, there may be a cross reaction with the heart which can cause thickening and deformity of the valve. In the case of the mitral valve, this may result in narrowing of the valve (mitral stenosis). The blood still flows in the usual direction described above but because of the narrowing, the pressure behind the narrowed, thickened valve is higher than it should be. People who have this problem may not even be aware of this and the problem may first be noted by a doctor with their stethoscope (listening to the heart hearing an abnormal sound or heart murmur) or when an echocardiogram is performed.
Symptoms
Symptoms of this condition include:
- shortness of breath
- swelling of the ankles
- irregular heartbeat
Diagnosis
The echocardiogram can show how much the valve is narrowed. The echocardiogram can also help determine if something needs to be done to fix the problem such as a surgical operation or whether the problem can be fixed by a less invasive procedure using a balloon to open up the narrowed valve.
Treatment
Heart valve surgery or special balloon procedures may be recommended to treat mitral stenosis that is severe enough to cause symptoms.
Prosthetic heart valves
The human heart has four valves that maintain blood flow in one direction by opening and closing in response to the change in pressure between the different chambers of the heart.
1. The mitral valve separates the left atrium from the left ventricle and allows blood flow from the left atrium to the left ventricle.
2. The aortic valve is situated between the aorta and the left ventricle and allows blood flow from the left ventricle into the aorta.
3. The tricuspid valve separates the right atrium from the right ventricle and allows blood flow from the right atrium to the right ventricle.
4. The pulmonic valve connects the right ventricle to the pulmonary artery and allows blood flow from the right ventricle into the pulmonary artery.
When one of these valves becomes severely diseased and causes symptoms such as shortness of breath or heart failure it is usually repaired or replaced (with an artificial valve, also known as prosthetic valve). This usually requires open heart surgery. There are various devices (such as rings, clips or prosthetic valves) that are used to repair or replace any of the heart valves although the most common valves affected are the mitral and aortic valves.
Prosthetic valves can be either mechanical (made of manmade, usually metallic material) or bioprosthetic (made of animal tissue)
1. Mechanical valves: Typically all of them have a ring at the centers of which there are discs (one or two) attached, that open and close in a fashion similar to the native valve and allow blood flow in only one direction.
2. Bioprosthetic (or tissue) valves can be either an entire pig valve, which is excised and preserved, or constructed from the pericardium (the lining around the heart) of either cow or pig. The bioprosthetic valves also have a ring from which their cusps are suspended.
All prosthetic valves (tissue valves and mechanical valves) are subject to normal wear and tear with time (typically 10-15 yrs or more) and can become dysfunctional. Similar to the native valves the prosthetic valves can develop either stenosis (blockage) or leaking (regurgitation). In addition, mechanical valves are prone to developing blood clots on their surface; this can be prevented by administrating blood thinners. Patients with prosthetic valves are also at increased risk for developing heart valve infection or endocarditis
Patients who develop prosthetic valve dysfunction develop symptoms very similar to native valve dysfunction including progressively increasing shortness of breath and heart failure. Patients are also at risk for stroke if a clot or an infectious mass (endocarditis) formed on the surface of the prosthetic valve gets dislodged and migrates to the brain. Echocardiography, both transesophageal and transthoracic can be extremely helpful in diagnosing abnormalities of prosthetic valves including endocarditis and can also be helpful in assessing the severity of this dysfunction and the need for further intervention such as repeat valve replacement surgery.
Pulmonary Stenosis
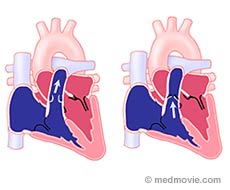 Background
Background
The pulmonary valve connects the right ventricle to the pulmonary artery. The pulmonary artery delivers blood to the lungs where it becomes oxygenated (“red blood”). The pulmonary valve usually has three leaflets. When there is narrowing of this connection there is blockage or obstruction of blood flow to the lungs. This is termed pulmonary stenosis. Pulmonary stenosis can occur in the right ventricle itself , at the level of the valve in the pulmonary artery or farther out where the pulmonary artery branches into two separate arteries that go to the right and left lungs. Valvar pulmonary stenosis accounts for 9-10% of all heart defects that are present at birth (congenital heart defects). Pulmonary stenosis at any level can also occur frequently with other heart defects.
Diagnosis
A heart murmur (abnormal heart sound heard with a stethoscope) is the most common finding with pulmonary stenosis. A transthoracic echocardiogram is the most reliable method for identifying the exact location of the narrowing, and determining how severe the narrowing or blockage is.
Specific findings on the echocardiogram in a patient with pulmonary stenosis will include:
- Size and appearance of the pulmonary valve
- Exact location of the narrowing
- Severity of the narrowing
Symptoms
Most children with milder forms of pulmonary stenosis have no real symptoms and are identified in the doctor’s office due to the finding of a heart murmur. With more severe levels of stenosis patients can have chest pain, fatigue, palpitations (“funny” or irregular heartbeats), shortness of breath, or difficulty exercising.
Treatment
Pulmonary stenosis is often very well tolerated, and if only a mild gradient is present, does not require any treatment. Your physician will determine if any treatment is necessary based on the echocardiogram results and any symptoms you may have (fatigue, difficulty exercising, or palpitations).
For patients with more severe pulmonary stenosis (gradient above 50mmHg) your physician may recommend relieving the narrowing. If the narrowing is at the level of the pulmonary valve, immediately above the valve level, or in the branch pulmonary arteries very close to the valve, a balloon valvuloplasty/angioplasty can be performed in the cardiac catheterization laboratory with excellent results.